1 Which of the Following Hydrocarbons Have Sigma Bonds
Identify the correct statement which is related to aromatic hydrocarbon a It has only sigma bonds b It has only pi bonds c It has a sigma and two pi bonds d It has a sigma and delocalized pi bond Answer. Next we have benzene.

Solved Question 37 1 Point Which Of The Following Chegg Com
Now there are three different types of hydrocarbons and those are alkanes alkenes and alkynes.

. Contains only pi bonds Contains only sigma bonds C Contains a sigma bond and delocalized pi bond Contains a sigma bond and two pi bonds d Question 38 1 point Substituent names are formed from by replacing the ane in the alkane name by ol b ene с. Are pi bonds stronger than sigma bonds. The first compound that was categorized as an aromatic hydrocarbon was benzene.
What bonding is in alkenes. Organic chemistry tricks jee neetIn this video you can easily find no. That is why alkenes undergo electrophilic addition reactions.
Acyclic four carbon atoms no multiple bonds. So here we have one sigma and two pi bonds in the middle. They are made up of a single sigma bond.
The correct option is. Any electrophile can come and attack it. The electrons of the π bond are delocalised and is thus a source of electrons.
Then we have a hydrogen attached to each of the S P too high priced carbon. Acyclic six carbon atoms three double bonds. Write the molecular formula for hydrocarbons with each of the following structural features.
Alkenes contain a double bond that is composed of one sigma and one pi bond between two carbon atoms. Carbons with sp3 hybrid atomic orbitals can in basic terms have sigma bonds that are molecular orbitals. The compounds possessing aromatic character show the following characteristics.
So we know alkanes are saturated and are connected with one sigma. Each carbon atom belonging to the benzene ring has two carbon-carbon sigma bonds one carbon-hydrogen sigma bond and one double bond with a neighbouring carbon in which the pi electron is delocalized. Aromatic hydrocarbons are also referred to as arenes.
SelectedDec 19 2018by Vikash Kumar. Hydrocarbons may contain sigma bonds andor pi bonds. Propane C3H8 has 2CC and 8CH bonds.
Each of the alkane member has its carbon in SP3 hybridized state in which all carbon atoms are tetrahedrally bonded with a bond angle ranging from 10928 and 25 S-orbital character and 75 P-orbital character. 2σ bonds and 2π bonds. Cyclic seven carbon atoms two double bonds.
Question 37 1 point Which of the following statement is correct about aromatic hydrocarbons. The second carbon-carbon bond is formed when the unhybridized 2p orbitals of the carbon overlap sideways. The double bond contains one strong sigma a bond and one weak Pi π bond.
See below free multiple-choice questions for Class 11 Hydrocarbons. B Bond energies of sigma- and pi-bonds are of the order of 264 kJmol and 347 kJmol respectively. It is also the most complex aryl hydrocarbon.
Unsaturated hydrocarbons have one or more double or triple bonds between carbon atoms. So starting with alkanes these are made up of saturated hydrocarbons or chains with all sp3 hybridization meaning they have single bonds are saturated and connected with one sigma bond. How many pi 1 electrons are there in the following molecule.
Of sigma and pi bonds of open chain hydrocarbons in just 1 second. A 0 b 1 c 2 d 3 2. Butene-1 may be converted to butane by reaction with.
Adarshthakur11 Adarshthakur11 Number of sigma bonds17 Number of pie bonds 4. Alkanes alkenes and alkynes are examples of aliphatic hydrocarbons. Alcoholic solution of KOH is used for.
Those with double bond are called alkenes and those with one double bond have the formula CnH2n assuming non-cyclic structures. Cyclic five carbon atoms one double bond. Ii Their molecular formulae suggest these compounds to be highly unsaturated due to the presence of one or more double bonds in the ring but they must behave as saturated compounds.
Hope it helps you New questions in Chemistry. Propene also known as propylene or methyl ethylene is an unsaturated organic compound with the chemical formula C 3 H 6 displaystyle ce C3H6. 10 sigma bonds in total.
Do carbon skeletons have double bonds. Is propene a single or double bond. 2 2 4.
I The compounds must be cyclic in nature and have flat planar structure. An aromatic hydrocarbon always has a sigma as well as a delocalized pi bond. A pi bond is a weaker chemical covalent bond than a sigma bond since π bonds have a smaller overlap between the orbitals but when it is put with a sigma bond it creates a much stronger hold.
Of sigma σ bonds to decide the state of hybridization of the Carbon. The bonding between the carbon atoms of ethylene results partially from the overlap of one sp² hybrid orbital of each carbon. By practicing these MCQ Questions for Class 11 Chemistry you will be able to revise the entire course and also test your understanding.
We know C - C 347 kJmol. Which of the following statements concerning valence bond theory are correct. Alkanes have only single bonds alkenes contain a carbon-carbon double bond and alkynes contain a carbon-carbon triple bond.
We have been asked to find out the hybridization of carbon as 13 and 5 just count the no. This is precisely the relation for an alkene so this is the hydrocarbon that has a double bond in its carbon skeleton. The orientation of the two pi bonds is that they are perpendicular to one another see Figure 6 below.
Carbon skeletons are the backbones of organic molecules. Acyclic five carbon atoms one double bond. Correct option is D 654321.
Examples are methane ethane propane etc. Questions and Answers Aromatic Hydrocarbons 1. Which of the following hydrocarbons has a double bond in the carbon skeleton.
How many rings andor pi bonds are present in a hydrocarbon with the formula C6H10. C C 619 kJmol. The overlap between the S and P orbital is called pi bond.
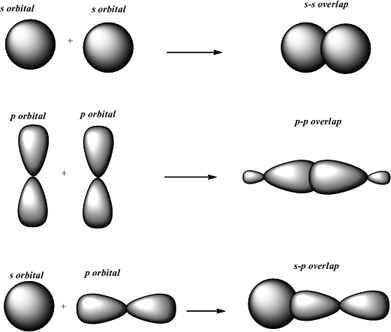
So instead of having one. HF is formed from the overlap of a hydrogen 1s orbital with a fluorine 2p orbital. The overlap of two S orbitals is called a sigma bond.
We do have a resident structure that is the equivalent because of the cemetery so you can see that you can move all of the electron density around and it looks pretty much identical.

How Many Sigma And Pi Bonds Are In This Molecule Study Com

Calculate The Number Of Sigma S And Pi Bonds In The Following Structures From Chemistry Hydrocarbons Class 11 Karnataka Board
Comments
Post a Comment